Our colloid products
include instructions for use. The experimental method is outlined below.
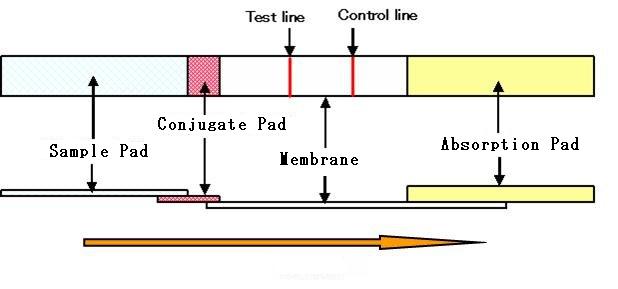
1. Immunochromatography kit: Structure
(1) Sample pad
Receives and distributes samples
uniformly onto a strip.
Operates as a filter.
(2) Conjugate pad
Maintains the gold colloid, antibody conjugate and the detection reagent
in a dry state. Releases the detection reagent quickly and uniformly to
the membrane.
(3) Membrane
Holds reagents (antibodies etc.) on the surface through the formation of
an immuno-complex. Controls the flow rate of the system.
(4) Absorbent pad
Absorbs the sample.
2. Swabbing of antibody on membrane
Materials
Membranes:
Membranes for immunochromatography may be purchased from manufacturers such as
Millipore. There are several types with different flow rates. The flow rate affects
detection sensitivity, measuring time, and the appearing of the unspecific
reaction
Antibody
for test lines: Antibody for the antigen to be detected. The operating concentration is
0.01?2 mg/mL (0.01?2mg/test).
If the antibody concentration is low, proteins such as BSA, which do not affect
the antigen?antibody reaction, are added to give a protein concentration of
about 0.5?1 mg/mL.
The antibody solution is diluted with phosphate buffer(s) (PB) (e.g., 10 mM PB (pH
7)).
Antibody for control lines: A secondary antibody to that conjugated with
the gold colloid. Example: anti-rabbit IgG antibody, anti-mouse IgG antibody.
Blocking
solution: Liquid in which proteins or polymers, such as gelatin, casein, BSA, IgG,
PVP, or PVA, are dissolved in buffer solution. Example: PBS solution containing
1% BSA.
Method
1. The
antibody solution is applied to the membrane in the form of a line.
(The position of the line depends on
the sensitivity of the reaction.)
2. The membrane is dried at room
temperature or at 37°C for 30 to 60 minutes.
3. The membrane is dipped in blocking solution for 10 to 30 minutes and shaken
gently.
4. The membrane is then dipped in weak buffer solution or distilled water,
shaken, and washed (2 to 3 times).
5. Any excessive moisture on the membrane surface is removed, and the membrane
is dried at room temperature or under vacuum.
Items to note
- Use of the membrane
- Amount of antibody solution to be used
- Use of blocking solution
- Dilution of the antibody solution
- Position of application of the antibody solution
3. Preparation ofgold-antibody conjugate
Materials
Gold
colloid: A colloid with a particle size of 50 nm is generally used. This may be purchased
from Winered Chemical Corporation.
The colloid is diluted with buffer
solution of a specific pH. On combining with an antibody, the pH may be
adjusted.
Antibody:
Antibody for the antigen to be detected. The antibody concentration after addition
to the gold colloid (OD525 = 1) is 50?500 ng/mL.
Blocking
solution: Contains proteins or polymers, such as gelatin, casein, BSA, IgG, PVP,
or PVA, in buffer.
Fiberglass:
Required for the conjugate pad. May be purchased from manufacturers such as
Millipore.
Stabilizing
solution for gold colloid: Low-salt buffer solution containing gold
colloid stabilizers such as BSA or PEG, along with added sugars such as sucrose
and/or trehalose.
Sugars are soaked in
the fiberglass, which increases the stability of the dried antibody.
Siliconized
tube: To prevent the gold colloid from sticking to the receptacle,
preparation of the gold-antibody conjugate
is carried out in a siliconized tube.
Items to note
- Amount of gold
colloid to be used
- Amount of antibody to be added
- pH at the time of antibody conjugation
- Composition of the blocking solution
- Composition of the gold colloid
suspension
This is known as the
“sandwich” system. Haptens can be detected by a competitive reaction system.
4. Example: Preparation of gold-antibody conjugate
Materials
Tris
buffer: The optimal pH varies depending on the antibody to be used; e.g., 5 mM
or 10 mM, pH 9.2.
Gold
colloid: WRGH1 (OD525 = 12) from Winered Chemical Corporation.
Antibody:Diluted
with Tris of appropriate concentration and pH (e.g., 5 mM, pH 9.2). Final
concentration 0.1~0.2 mg/mL or 30~60mg/mL.
1% BSA
solution: Prepared using 5mM Tris, pH 9.2.
BSA?PEG
mixed solution: Containing 1% BSA solution and 1% PEG solution, mixed at a ratio of 9:1.
Stabilizing
solution for gold colloid conjugate: Tris buffer of appropriate
concentration and pH (e.g., 5 mM, pH 9.2) is used. pH adjustment may not be
necessary. Because during conjugation reaction, the gold colloid may aggregate
in buffer solution in the presence of high concentration of salt, becoming a
purple-blue color, dialysis of salt may be required.
Method
The gold colloid
solution and the antibody solution are mixed in a 1:1 ratio and the mixture is
left to stand for 15 minutes.Example
For 100µL each of gold colloid and antibody solutions:
1. BSAPEG mixed solution (100µL) is added, the mixture is centrifuged at 5000 rpm for 5 minutes,
and the supernatant is removed.
2.BSA?PEG mixed solution (200µL)
is again added and mixing is carried out ultrasonically (about 30 seconds),
followed by addition of another 200µL of BSA?PEG mixed solution. The mixture is again centrifuged and
the supernatant is removed.
3. Stabilizing
solution (100µL) is added and mixing is carried out by Voltex
or ultrasound (10 seconds). Depending on the antibody used, the antibody
concentration and the pH of the buffer solution and stabilizing solution may
vary.
Bibliography
1. Masatoshi Watabe, Shigeaki Furukawa,
Suguru Akamatsu, and Tetsuya Oda, “Simple diagnostic technical development”,
Bio-industry, No. 10, Vol. 22, p60-65 (2005).
2. “Method of manufacture of
precious-metal colloids, precious-metals particles, composites, and
precious-metal particulates.” Masatoshi Watabe, Patent No. 4368855, Japan.
Patent international publication
number: WO 2005/023468 A1
3. Julian E. Beesley, “Colloidal Gold:
A new perspective for cytochemical marking”, Royal Microscopical Society
Handbook No. l7, Oxford Science Publications, Oxford University Press (l989).
4. Sadanori Yokota and Osamu Fujimori,
“Immunogold method: Immunohistochemistry with colloid gold”, Soft Science Company,
1992.
Manufacturing and selling company: Winered Chemical Corporation
246-4 Inume-machi, Hachioji, Tokyo 193-0802, Japan
TEL: (81)42-624-3755 FAX: (81)42-624-3732 URL: www.winered.jp e-mail: mawatabe@nifty.com